lewis structure ethyne|C2H2 Lewis Structure Tutorial : Pilipinas Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. . Find our live Invesco Global Opportunities Fund Class R fund basic information. View & analyze the OGINX fund chart by total assets, risk rating, Min. investment, market cap and category.
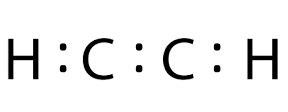
lewis structure ethyne,In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Ethyne or Acetylene. Note that we'll need a triple bond between the Carbon .Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. . In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like C2H2.
397K views 13 years ago. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene). For the C2H4 structure use the periodic table to find the total number of valence .Learn more. The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding .Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair .
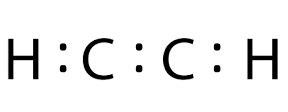
A. Definition and concept. The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves .
Objectives. After completing this section, you should be able to. account for the formation of carbon-carbon double bonds using the concept of sp2 hybridization. describe a carbon .Bonding in Ethane. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, .lewis structure ethyne C2H2 Lewis Structure Tutorial C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms .lewis structure ethyne Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to . Ethene or C2H4 is a common straight-chain acyclic alkene and an important member of organic hydrocarbons. Having a double C=C bond, it is unsaturated and this gives rise to several properties. Here, we learned about how to draw the proper Lewis Structure and find out the molecular geometry of an ethylene molecule.Drawing the Lewis Structure for HCCH. HCCH (Ethyne) can also be written as C 2 H 2. Ethyne is also called Acetylene. For the HCCH Lewis structure you'll need to form a triple bond between the two carbon atoms. Hydrogen atoms only need two electrons for a full outer shell. There are a total of 10 valence electrons for the HCCH Lewis structure. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val.
Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru.
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms .Ethylene (commonly knows as ethene), CH 2 CH 2, is the simplest molecule which contains a carbon carbon double bond. The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule have been .The correct Lewis structure for ethene is shown below: In the molecule ethene, both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped probability areas above and below the plane of the six atoms.Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C2H2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. .
A. Chemical formula of Ethyne. The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. II.
The simplest member of the alkyne series is ethyne, C 2 H 2, commonly called acetylene. The Lewis structure for ethyne, a linear molecule, is: The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, CH 3 CH 2 C ≡ CH CH 3 CH 2 C ≡ CH is called .
H. 4. ) Lewis Structure, Hybridization. Ethene's lewis structure can be built by VSEPR rule. Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. Ethylene is a colorless gas with the chemical formula C2H4. It is a hydrocarbon with two carbon connected with a double bond. In this article, we will study ethene (C2H4) lewis structure, molecular geometry, .
Ethene is the formal IUPAC name for H 2 C=CH 2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group ( CH2CH3 C H 2 C H 3) but there is a double bond between the two carbon atoms in it. Ethene has the formula C2H4 C 2 H 4 and is the simplest alkene because it has the .For C 2 H 4 you have a total of 12 total valence electrons. Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only .C2H2 Lewis Structure Tutorial An orbital view of the bonding in ethyne. Ethyne is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1).The carbon atom does not have enough unpaired electrons to form four bonds (1 to the hydrogen and three to the other carbon), so it needs to promote one of the 2s 2 pair into the empty 2p z orbital. This is .
lewis structure ethyne|C2H2 Lewis Structure Tutorial
PH0 · Lewis Structure for C2H2 (Ethyne)
PH1 · How to Draw the Lewis Structure for C2H4
PH2 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH3 · C2H2 Lewis Structure, Geometry
PH4 · C2H2 Lewis Structure Tutorial
PH5 · C2H2 Lewis Structure
PH6 · C2H2 (Ethyne) Lewis structure
PH7 · C2H2 (Acetylene
PH8 · 1.8: sp² Hybrid Orbitals and the Structure of Ethylene
PH9 · 1.13: Ethane, Ethylene, and Acetylene